Alphtil
Chemical name:
Tilmicosin (IUPAC name): (10E,
12E)-(3R,4S,5S,6R,8R,14R,15R)-14-(6-deoxy-2,3-di-O-methyl-b-d-allo-hexopyranosyoxymethyl)-5-(3,6-dideoxy-3-dimethylamino-b-d-gluco-hexapyranosyloxy)-6-[2-(cis-3,5-dimethyl-piperidino)ethyl]-3-hydroxy-4,8,12-trimethyl-9-oxoheptadeca-10,12-dien-15-olide
Chemical Abstracts Services Name: tylosin, 4A-O-de(2,6-dideoxy-3-C-methyl-alpha-L-ribo-hexopyranosyl)-20-deoxy-20-(3,5-dimethyl-1-piperidinyl)-(20(cis:
trans))
C.A.S. number: 108050-54-0
Synonyms: 20-Deoxy-20-(3,5-dimethylpiperidin-1-yl)-desmycosin
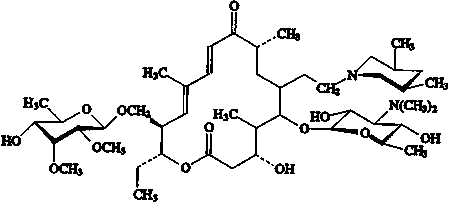
Molecular formula:
C46H80N2O13
Molecular weight:
869.15
OTHER INFORMATION ON IDENTITY AND PROPERTIES
Pure active ingredient:
Melting point: Not determined
Solubility:
Freely soluble (1500 mg/L or greater)
in organic solvents (hexane, acetone, acetonitrile, chloroform,
dichloromethane, ethyl acetate, methanol, tetrahydrofuran);
water solubility is temperature and pH dependent, but is 566
mg/mL at pH 7 and 25ˇăC.
Purity:
Tilmicosin consists of 82-88% cis
isomer and 12-18% trans isomer, as determined by liquid
chromatographic assay.
RESIDUES IN FOOD AND THEIR EVALUATION
CONDITIONS OF USE
General
Tilmicosin is a macrolide antibiotic developed for
veterinary use. It is recommended for treatment and prevention
of pneumonia in cattle, sheep and pigs, associated with Pasteurella
haemolytica, P. multocida, Actinobacillus pleuropneumoniae,
mycoplasma species and other microorganisms found sensitive
to this compound. Tilmicosin has not been previously reviewed
by the Committee.
Dosage
Available formulations of tilmicosin include an
injectable for use in cattle and sheep (Micotil 300) and premix
formulations for swine (Pulmotil G40, G100 and G200). The
recommended dose of the injectable formulation in both cattle
and sheep is a single subcutaneous (SC) injection of 10 mg/kg
BW. Recommended dose for swine in feed is 200-400 mg/kg of
feed for 10 to 21 days, equivalent to 8-20 mg/kg BW per day.
METABOLISM
Pharmacokinetics
General
Rat
Thirty Fischer strain 344 rats (15 male, 15 female)
each received an oral dose of 20 mg/kg BW 14C-tilmicosin
on three successive days (Donoho, 1988). A separate group
(10 males, 10 females) served as controls. Excreta were collected
for 2 days prior to dosing, during the 3 days on which doses
were administered and for 3 days following the last dose.
Urine and faecal samples were pooled separately for the males
and the females for each sampling day. All rats were sacrificed
3 days after the final dose was administered and livers from
the treated rats were collected and pooled by sex. Livers
from the non-treated rats were combined to provide a single
control pool. Urine collected from rats 48 hours after treatment
began contained residues equivalent to 10 mg/L tilmicosin,
shown to be primarily parent compound by radiochromatography
and by TLC autoradiography. Faeces contained approximately
35 mg/kg tilmicosin equivalents, found to be a metabolite
designated as T-1, parent tilmicosin and a tilmicosin-related
compound, designated as T-2. Rat livers, however, contained
primarily parent
tilmicosin
and practically no T-2, indicating that this compound is not
bioavailable to animals when given orally. T-2 was isolated
from technical grade tilmicosin and a structure has been proposed,
based on mass spectral and NMR data, based on a molecular
formula of C84H143N3O26,
and a molecular weight of 1609. T-1 was identified as N-desmethyl
tilmicosin, corresponding to a loss of-CH2, apparently
on the mycaminose sugar of tilmicosin. T-1 has a molecular
weight of 854 and a composition of C45H78N2O13.
Twenty Fischer strain rats (10 male, 10 female)
were given an oral dose by gavage of 50 mg/kg BW tilmicosin
per day for 5 successive days (Donoho and Kennington, 1993).
Urine and faeces were collected and pooled by sex. Faeces
were found to contain a metabolite designated as T-4, previously
identified in pig faeces (Donoho et al, 1992). The
common identity of the metabolites isolated from the two experiments
was confirmed by LC/MS/MS.
Cattle
No difference in absorption was observed in calves
given a single dose of 10 mg/kg BW of tilmicosin by IM injection
in the semitendinosus muscle or SC in the dorsolateral chest
or lateral neck muscles (Thomson, 1989a), or in feedlot cattle
which received a similar treatment (Thomson, 1989b). Peak
mean tilmicosin levels were observed in 1-hour serum samples
in the calves, but were close to peak for 12 hours post-dosing
in the cattle, with Tmax for the recommended dorsolateral
chest muscle site being 6 hours. In a study in which a single
steer received one dose of 30 mg/kg BW of 14C-tilmicosin,
recovery of radiolabelled material within the first 7 days
was 16.0% in urine and 61.0% in faeces (Giera et al.,
1986a). At slaughter 15 days post-treatment, 90.8% of the
radiolabelled material had been eliminated in urine and faeces,
while 3.8% remained in the injection site and smaller amounts
in the liver and kidney (residues equivalent to 9 and 18 mg/kg,
respectively). These results were confirmed in a second experiment
in which a steer received a single dose of 20 mg/kg BW 14C-tilmicosin.
It was shown that 22.7% of the radiolabelled material was
eliminated in the urine and 63.5% in the faeces within 7 days
of treatment (Giera et al., 1986b). At 21 days post
treatment, 91.8 % of the radiolabelled material had been recovered
in urine and faeces, while 0.3 % was found in the liver, kidney
and injection site. The data from these studies suggest a
slower absorption in mature feedlot cattle than in neonatal
calves.
Further studies to characterize the recovered radiolabelled
material indicated that the majority was parent compound (Giera
and Peloso, 1986). As in the rat, the three primary substances
found in the liver of cattle were parent compound, T-1 and
T-2, but a minor metabolite designated T-3 was also found
in cattle faeces (Donoho, 1988). This metabolite appeared
to be formed by the replacement of -N(CH3)2
on the mycaminose sugar with -OH. It has also been shown that
tilmicosin residues are distributed throughout the body of
a steer following a single SC injection of 20 mg/kg BW, with
highest persistent levels in the liver and the injection site
at 21 days (5.5 and 5.2 mg/kg, respectively), but significant
residues also occurring in the kidney (2.3 mg/kg) and lung
(0.9 mg/kg) (Giera et al, 1986b).
Sheep
The absorption of tilmicosin in sheep was the subject
of three reported studies. In the initial study, 3 groups,
each consisting of 5 sheep weighing approximately 40 kg, received
intravenous doses of tilmicosin at rates of 2.5, 5.0 and 7.5
mg/kg BW and were then observed for toxic responses (Cochrane
and Thomson, 1990). Allowing a minimum 14 days between treatments,
the same animals were also treated with SC injections in the
dorsolateral chest of tilmicosin at 10, 30, 50 and 150 mg/kg
BW, with the same group being used for the 10 and 150 mg/kg
dose rates, observing a 14 day period between treatments.
Injection sites were clinically detectable at dose rates of
30 mg/kg BW and higher, with the time period in which they
were observable increasing with the dose rate. Pharmacokinetic
parameters determined for the various treatment levels are
given in Table 1.
Table 1. Pharmacokinetics (serum) of tilmicosin in sheep following
a single SC injection in the dorsolateral chest
|
Variables
|
Dose
Rate (mg/kg BW) |
|
10.0
|
30.0
|
100.0
|
150.0
|
|
Cmax
(m g/mL) |
0.44
|
1.14
|
2.15
|
2.50
|
|
tmax (h) |
8
|
12
|
24
|
36
|
|
AUCa
(m
g/h/mL) |
10
|
35
|
120
|
185
|
In a second study, two groups of 6-month old sheep
(28-50 kg BW, 24 animals per group) received doses of tilmicosin
in the left dorsolateral chest wall at 10 and 20 mg/kg BW,
respectively (Patel et al, 1991). Serum samples were
collected from 4 animals from each treatment group prior to
dosing and at intervals of 8, 24, 48, 72 and 96 hours post-dosing.
Samples were analyzed using a liquid chromatographic assay
with a limit of determination of 0.05 mg/L. Highest serum
concentrations were observed in the 8-hour samples for both
treatment groups (1.18 and 2.28 mg/L, respectively), with
depletion in blood apparently following a 2-compartment model.
Finally, 14 lambs (7 male, 7 female, BW 16-23
kg), received a single SC injection of 14C-tilmicosin at a dose of 20 mg/kg BW, administered in the
lateral thoracic wall (Hawkins et al, 1993). Collection
of urine and faeces revealed excretion of 85.2% of the dose
within 7 days, with 71.9% being in the faeces. Residues were
distributed throughout tissues assayed in animals sacrificed
at days 3 through 28, with highest levels found in liver and
injection site skin at day 28. The residue appeared to be
predominantly parent tilmicosin, with T-1 and T-2 also being
found, similar to results observed in cattle. Based on assay
of serum samples by liquid scintillation counting, Cmax
was 1.42 mg/L at Tmax of 3.8 hrs. Analysis of these
samples by liquid chromatography for parent tilmicosin gave
a Cmax of 0.96 mg/L at Tmax of 4 hrs
(Patel et al, 1992).
Swine
Due to toxic response to intravenous bolus dosing,
the basic pharmacokinetic parameters for swine could not be
determined using this experimental approach. In 10-week old
pigs administered tilmicosin at 200 and 400 mg/kg in feed
(approximately 11 and 21 mg/kg/day dose levels), serum and
lung tissue samples were collected post-mortem for groups
of 4 animals (2 male, 2 female) slaughtered at 2, 4, 7, 10
and 14 days after initiation of treatment (Thomson, T.D.,
Darby, J.M., Moran, J.W., and Tonkinson, L.V., 1993). Serum
levels were low, below the limit of quantitation (0.1 mg/L)
in 17 of 20 animals on the low dose feed. At the higher dose
rate, tilmicosin concentrations in serum ranged from <
0.10 to 0.23 mg/L, with detectable levels in 17 of 20 animals.
Residue concentrations in lung tissue increased between 2
and 4 days of treatment, but then remained relatively stable
at both treatment levels. At the lower treatment rate, concentrations
in lung tissue were in the range 1.1 to 1.4 mg/kg, while at
the higher rate levels were approximately 2.2 mg/kg.
Three studies were reported in which the excretion
of tilmicosin residues by orally dosed swine was investigated.
In the initial study, 14C-tilmicosin,
labelled in the piperidine ring, was administered in a single
dose in feed fortified at 220 mg/kg, after which urine and
faeces were collected during a 13-day withdrawal period (Giera
and Thomson, 1986). Overall, 80% of the radiolabelled material
was recovered in the faeces and 15% was in the urine. However,
there was concern that results may have been affected by a
contaminant, which accounted for 6% of the residue in urine.
A second study, using a dose of 110 mg/kg in feed, provided
recoveries of 75.6 % and 62.3 % in faeces collected from 2
hogs over 11 days following a single treatment, while recoveries
in urine were 3.9% and 4.9%, respectively (Donoho and Thomson,
1988). More than 90% of the recovered radioactivity was found
within 3 days of dosing. In a third study, 6 pigs were treated
with feed containing 400 mg/kg 14C-tilmicosin
for 5 days and urine and faeces from 2 pigs slaughtered at
14 days withdrawal were collected for analysis (Donoho et
al, 1992). Recovery was 70.1 % of the original dose in
faeces (64.5%) and urine (5.6%), with most of this occurring
in the first 7 days following administration (62.2% of total
dose). The residues were found to be primarily parent tilmicosin,
with a small amount of metabolite T-1 in the urine and a metabolite
designated as T-4 accounting for 10 % of the residues in faeces.
T-4 was proposed to have a structure in which one carbon-carbon
double bond was reduced and -SO3H was added to
the macrolide ring, based on spectrometric analysis.
TISSUE RESIDUE DEPLETION STUDIES
Radiolabeled Residue Depletion Studies
Cattle
Five animals (4 steers, 1 bull, weights 157-202
kg) received a single dose of 20 mg/kg BW 14C-tilmicosin by SC injection in the dorsolateral rib area (Giera
et al, 1986b). Total radioactive residues were determined
in the primary edible tissues at withdrawal intervals of 3
(1 steer), 21 (2 steers) and 56 days (1 steer, 1 bull). Residues
were similar in liver and kidney tissues at day 3 (36.0 and
39.2 mg/kg, respectively), but were higher in liver at the
longer withdrawal periods. Highest concentrations of residue
were found in the 3-day injection site (81.6 mg/kg), but residues
in 21-day injection sites were similar to those found in the
matched livers. At 56 days, residues in injection sites were
lower than in the livers. Residues were at 2.0 mg/kg in muscle
tissue at day 3, but were not detected at the longer withdrawal
times. Results were consistent with those reported in a 15-day
withdrawal study with a single steer, using a dose of 30 mg/kg
BW 14C-tilmicosin,
where total residues found were: injection site, 88.4 mg/kg;
liver, 17.6 mg/kg; kidney, 8.9 mg/kg; muscle, 0.2 mg/kg (Giera
et al, 1986a).
A further experiment was conducted using 12 cattle
(each approx. 200 kg BW) which received a single SC injection
of 14C-tilmicosin
at a dose of 10 mg/kg BW over the rib cage (Donoho et al,
1988). The results, shown in Table 2, demonstrate a depletion
pattern similar to that found in the earlier studies. Significant
residues may remain at the injection site for 4-6 weeks post-injection.
Residues in the liver and kidney are similar 3 days after
treatment, but at longer withdrawal periods residues are more
concentrated in the liver, reflecting the observed distribution
of the drug residues found in faeces and urine. Residues found
in muscle and fat tissue are significantly lower than those
found in the organ tissues and injection sites.
Table 2. Residues of tilmicosin in tissues of cattle resulting
from a single SC injection of 14C-tilmicosin
at 10 mg/kg BW.
|
Withdrawal
(days) |
14C-Tilmicosin
Equivalents (mg/kg) |
|
n
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Inj.
Site |
|
3
|
2
|
19.44
|
18.09
|
0.40
|
0.24
|
73.53
|
|
14
|
2
|
11.63
|
2.51
|
0.09
|
0.05
|
13.82
|
|
28
|
3
|
5.74
|
0.59
|
<
0.05 |
0.03
|
5.07
|
|
42
|
3
|
3.52
|
0.27
|
NDa |
<
0.04 |
0.94
|
|
56
|
2
|
2.72
|
---b |
---b |
---b |
0.33
|
a
ND = not detected; b
--- = not analyzed
Liver, muscle and injection site muscle from these
animals was also analyzed for parent compound by HPLC, using
a method with a reported LOQ for liver and muscle of 0.06
mg/kg and recoveries of 60-80 %. The results, reported in
Table 3, showed that in liver, parent compound declined as
a percentage of total residue from 37% at 3 days withdrawal
to 7 % at 28 days. During the same period, about 50 % of the
total residue at the injection site is parent compound.
Table 3. Residues of tilmicosin parent compound in tissues
of cattle treated with a single SC injection equivalent to
10 mg/kg BW. Data were not corrected for recovery (recovery
of 60 - 80% reported).
|
Withdrawal
(days) |
Parent
Tilmicosin Concentrations (mg/kg) |
|
Liver
|
Muscle
|
Injection
Site |
|
3
|
7.11
|
0.18
|
42
|
|
14
|
1.99
|
<
0.05 |
8.3
|
|
28
|
0.38
|
---a |
2.6
|
|
42
|
<
0.10 |
---a |
---a |
|
56
|
<
0.06 |
---a |
---a |
a
--- = not analyzed
Sheep
A study in which the absorption and metabolism of
tilmicosin in sheep was investigated also reported the depletion
of the drug following SC administration at a dose of 20 mg/kg
BW (Hawkins et al, 1993). Fourteen ruminating lambs
(7 male, 7 female, 16-23 kg BW) were assigned to a control
group (2) or to the treated group (12). Animals were then
slaughtered at intervals of 3, 7, 21 and 28 days post-injection,
with the controls being slaughtered with the 7-day group.
Residues of tilmicosin, measured as equivalents by radioactivity,
were determined in the various edible tissues, as reported
in Table 4. Depletion followed a pattern similar to that found
in cattle, with most persistent residues found in liver and
rapid depletion of residues in muscle and fat tissues collected.
Total residues remained above 1 mg/kg in the injection site
at 28 days post-treatment.
Table 4. Residues of total tilmicosin in tissues of sheep treated
with a single SC injection of 14C-tilmicosin
at a dosage of 20 mg/kg BW.
|
Withdrawal(days)
|
Mean
14C-Tilmicosin
Equivalents (mg/kg) |
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Inj.
Site |
|
3
|
9.98
|
21.09
|
1.26
|
<
1.24 |
43.15
|
|
7
|
5.77
|
4.07
|
0.42
|
<
1.15 |
14.38
|
|
21
|
3.67
|
1.42
|
<
0.26 |
<
1.17 |
5.32
|
|
28
|
2.70
|
0.55
|
<
0.26 |
<
1.20 |
1.32
|
Tissues collected from the sheep in the above study
were also analyzed for parent compound using a liquid chromatographic
analysis with a limit of quantitation of 0.05 mg/kg (Patel
et al, 1993). Samples were stored at -20ˇăC and were
analyzed within several months of collection. Reported results,
as given in Table 5, were corrected for recovery using the
recovery of tilmicosin from a fortified sample included in
each analytical run. These results reflect the depletion pattern
for the total residue, with most persistent residues of parent
compound found in the liver and the injection site. They also
suggest that the majority of the residues found in liver and
the injection site 7 days or longer after treatment are not
parent compound. The nature and activity of these residues
is not fully known, but T-2 was found to form an increasingly
significant portion of the total residue (25-29%) at the longer
withdrawal times in liver.
Table 5. Residues of parent tilmicosin in tissues of sheep
treated with a single SC injection of 14C-tilmicosin
at a dosage of 20 mg/kg BW.
|
Withdrawal(days)
|
Mean
Residues Parent Tilmicosin (mg/kg) |
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Inj.
Site |
|
3
|
2.44
|
12.41
|
0.48
|
0.07
|
20.35
|
|
7
|
0.73
|
1.29
|
0.19
|
<
0.05 |
7.06
|
|
21
|
0.31
|
0.47
|
NDa |
NDa |
2.50
|
|
28
|
0.16
|
0.06
|
NDa |
NDa |
0.12
|
a
ND = not detected; analyzed by HPLC method with limit of quantitation
of 0.05 mg/kg.
Swine
Three barrows (15.5-18.0 kg BW) were used in a
preliminary study to determine the distribution of 14C-tilmicosin
in swine (Giera and Thomson, 1986). Two animals received a
single dose of 14C-tilmicosin
in feed fortified at 220 mg/kg (approx. dose 5 mg/kg BW),
while the third animal served as a control. The animals were
slaughtered at 13 days post-treatment, at which time total
residues, as determined by radioactivity, were: liver, 0.07
mg/kg; kidney, 0.08 mg/kg; muscle and fat, < 0.02 mg/kg.
In a subsequent study, six pigs (3 male, 3 female, approx.
22 kg BW) received a diet containing 400 mg/kg 14C-tilmicosin
for
twice daily for 5 days (estimated dose 18 mg/kg BW/day). Two
additional animals served as controls. Pairs of the treated
animals were slaughtered at withdrawal times of 0, 7 and 14
days, and liver, kidney, muscle and fat were collected for
analysis by total radioactivity and by HPLC. The results of
these analyses, shown in Table 6, demonstrate a similar distribution
to that observed in cattle and sheep, with highest persistent
residues being found in the liver. Parent compound appears
to be the most significant residue (It should be noted that
the HPLC assay results are not recovery corrected. Recovery
for the HPLC method used is reported to be in the 85-90% range).
A similar study was conducted in which nine 2-month-old
pigs (3 barrows, 6 females, approx. 17 kg BW) received feed
containing 600 mg/kg 14C-tilmicosin
for 5 successive days, for an estimated dose 23 mg/kg BW/day
(Donoho et al., 1991). Similar groups each consisting of 3
pigs were slaughtered at withdrawal times of 6 hrs (0 days),
14 and 28 days. An untreated pig was used as a control. Tissue
samples collected at slaughter were analyzed by total radioactivity
and by HPLC, as in the previous experiment (Table 6).
Other Residue Depletion Studies (with unlabelled drug)
Cattle
Twelve cattle (8 steers, 4 heifers, approx. 200
kg BW) each received a single SC injection of tilmicosin in
the neck at a dose rate of 10 mg/kg BW (Peloso and Thomson,
1988). Groups consisting of two steers and 1 heifer were slaughtered
at each of 14, 28, 35 and 42 days post-treatment and samples
of edible tissues were collected for analysis by an HPLC method
with an LOQ of 0.05 mg/kg. The data were not corrected for
recovery, which was in the range of 80% or higher for all
tissues and concentrations tested. The results, given in Table
7, demonstrate, as in other studies, that highest residues
are found at the injection site and in liver tissue. While
the results for residues of parent compound were generally
higher at 14 and 28 days in the study using the same dose
rate with 14C-tilmicosin in cattle of similar weight (see Table 3), the
overall depletion patterns are similar.
Table 6. Total residues determined by radioactivity and residues
of parent tilmicosin, determined by HPLC, in pigs which received
a feed containing 400 or 600 mg/kg 14C-tilmicosin
for 5 successive days.
|
Withdrawal
Time (days) |
Dose
Rate (mg/kg) |
Mean
Tilmicosin Residue (mg/kg)a
|
|
|
Assay
|
Liver
|
Kidney
|
Muscle
|
Fat
|
|
0
|
400
|
RA
|
4.55
|
4.31
|
0.39
|
0.12
|
|
HPLC
|
2.33
|
2.34
|
0.24
|
0.13
|
|
600
|
RA
|
10.62
|
12.28
|
1.09
|
0.41
|
|
HPLC
|
9.86
|
12.98
|
1.00
|
0.44
|
|
7
|
400
|
RA
|
1.42
|
0.70
|
<
0.02 |
0.02
|
|
HPLC
|
0.75
|
0.35
|
<
0.05 |
<
0.05 |
|
14
|
400
|
RA
|
0.38
|
0.16
|
<
0.02 |
<
0.01 |
|
HPLC
|
0.19
|
0.09
|
<
0.05 |
---
|
|
600
|
RA
|
1.58
|
0.58
|
<
0.10 |
<
0.06 |
|
HPLC
|
1.04
|
0.41
|
<
0.05 |
<
0.05 |
|
28
|
600
|
RA
|
0.32
|
0.15
|
<
0.10 |
<
0.06 |
|
HPLC
|
0.14
|
0.07
|
---
|
---
|
a
For radioactivity assay, LOD's were 0.02 and 0.01 mg/kg for
fat and muscle, respectively, in the 400 mg/kg treatment,
and 0.10 and 0.06 in the 600 mg/kg treatment; LOD for fat
and muscle by HPLC assay was 0.05 mg/kg; --- indicates sample
not analyzed.
Table 7. Residues of tilmicosin parent compound in tissues
of cattle treated with a single SC injection equivalent to
10 mg/kg BW.
|
Withdrawal(days)
|
Mean
Residues Parent Tilmicosin (mg/kg) |
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Inj.
Site |
|
14
|
0.93
|
0.94
|
<
0.05 |
<
0.05 |
18.94
|
|
28
|
0.26
|
0.14
|
<
0.05 |
<
0.05 |
2.92
|
|
35
|
0.18
|
0.11
|
<
0.05 |
---a |
0.78
|
|
42
|
<
0.09 |
<
0.06 |
---a |
---a |
0.29
|
a
--- = not analyzed.
Sheep
Twenty-eight sheep (Swaledale, 26.2-51.2 kg BW)
were acclimatized for 1 week to assess health status prior
to a single administration of 10 mg/kg BW tilmicosin by SC
injection into the left dorsolateral chest wall (Patel et
al, 1995). The sheep, which had been divided prior to
injection, into groups of 4 animals (2 male, 2 female) were
sacrificed at 14, 21, 28, 35, 42 and 49 days post-dosing.
A group of 4 control sheep was also slaughtered at day 14.
Samples collected at slaughter included the whole liver, both
kidneys, thigh muscle (500 g), renal fat (200 g) and the injection
site. The latter was collected by removing a 15 cm diameter
area (or greater) around the point of injection to provide
500 g of edible tissue. Samples were stored at -20ˇăC until
assayed, within several months of collection, using a liquid
chromatographic assay with a limit of quantification of 0.05
mg/kg. Results, reported in Table 8, were corrected for recovery
using fortified samples included in each analytical run.
Table 8. Residues of parent tilmicosin in tissues from sheep
administered a single SC dose at 10 mg/kg BW.
|
Withdrawal
(days) |
Mean
Parent Tilmicosin Concentration (mg/kg) |
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Inj.
Site |
|
14
|
0.11
|
0.16
|
NDa |
< 0.05b
|
1.53
|
|
21
|
0.07
|
0.07
|
ND
|
<
0.05 |
0.14
|
|
28
|
<
0.05 |
<
0.05 |
ND
|
ND
|
0.08
|
|
35
|
<
0.05 |
<
0.05 |
ND
|
ND
|
<
0.05 |
|
42
|
<
0.05 |
<
0.05 |
ND
|
ND
|
ND
|
|
49
|
<
0.05 |
<
0.05 |
ND
|
ND
|
<
0.05 |
a
ND = not detected.
b
< 0.05 indicates some or all samples in group were below
LOQ of 0.05 mg/kg; each such group may include samples which
were ND.
Swine
Thirty finisher swine (15 male, 15 female, approx.
60 kg BW at start of experiment) were fed a diet containing
400 mg/kg tilmicosin for 21 days (Readnour and Darby, 1993).
Groups, equally divided by sex, were slaughtered at withdrawal
times of 0, 7, 14 and 21 days (0 days = 6 hrs). Samples of
liver, kidney, muscle, fat and skin were collected from each
animal and analyzed using an HPLC method with a limit of quantitation
of 0.02 mg/kg. Untreated control animals were killed about
1 hr before slaughter of the zero withdrawal group. The assay
results, shown in Table 9, demonstrated as in previous studies
that highest persistent residues are found in the liver. Results
reported were not corrected for recovery, but the method specifies
a minimum recovery of 70%, which was monitored by inclusion
of a fortified sample in each analytical run.
Table 9. Residues of parent tilmicosin in tissues of swine
following administration at 400 mg/kg in feed for 21 days
(equivalent to approximately 20 mg/kg BW/day).
|
Withdrawal
(days) |
n
|
Mean
Tilmicosin Residues (mg/kg) |
|
Liver
|
Kidney
|
Muscle
|
Fat
|
Skin
|
|
0
|
12
|
4.16
|
4.14
|
0.32
|
0.09
|
0.08
|
|
7
|
6
|
0.71
|
0.34
|
<
0.02 |
<
0.02 |
0.12
|
|
14
|
6
|
0.19
|
0.08
|
<
0.02 |
<
0.02 |
0.05
|
|
21
|
6
|
0.06
|
0.06
|
---a |
---a |
<
0.02 |
a
--- = not analyzed
Milk
Milk was analyzed from 4 ewes which each received
a single injection of 10 mg/kg BW tilmicosin in the dorsolateral
chest (Patel et al, 1992). On the day of treatment,
milk was collected 8 hours following injection, while subsequent
collections were at regular morning and afternoon milkings.
Milk collected at each milking on days 1 and 2 was treated
as separate samples, while milk from the two milkings of each
animal was combined on subsequent sampling dates. Samples
were homogenized and stored at -20ˇăC until analyzed, using
the Delvotest P and an HPLC assay. Control milk was obtained
from untreated animals. All milk samples collected were positive
using the Delvotest from days 0 through 6. One sample gave
a full inhibition result on day 7, while the other 3 showed
partial inhibition. Slight inhibition was seen in samples
collected on days 8 and 9 and in two samples on day 12. However,
HPLC analysis of the samples giving slight or partial inhibition
revealed levels of tilmicosin that were below the claimed
LOD of the Delvotest assay kits, 0.15 mg/L. All other samples
collected daily through day 28 were negative. Results of the
Delvotest and HPLC assays are shown in Table 10.
Table 10. Residues of parent tilmicosin in sheep's milk following
a single SC administration at 10 mg/kg BW, as determined by
HPLC and Delvotest Assays.
|
Time
Post-Treatment |
Delvotest
Positive a
|
Mean
Tilmicosin Residue (mg/L)b
|
|
8
h |
4/4
|
10.25
|
|
23
h |
4/4
|
9.56
|
|
30
h |
4/4
|
7.86
|
|
47
h |
4/4
|
2.82
|
|
54
h |
4/4
|
1.97
|
|
3d
|
4/4
|
1.16
|
|
4
d |
4/4
|
0.49
|
|
5
d |
4/4
|
0.27
|
|
6
d |
4/4
|
0.13
|
|
7
d |
1/4
|
0.12
|
|
8
d |
0/4
|
0.11
|
|
9
d |
0/4
|
0.09
|
|
10
d |
0/4
|
0.06
|
|
14
d |
0/4
|
<
0.05 |
|
21
d |
0/4
|
<
0.05 |
a
Samples classed as positive gave full inhibition.
b
LOQ = 0.05 mg/L.
One study has been reported in which six dairy cows
each received a single SC injection of 10 mg/kg BW tilmicosin
(Helton-Groce et al., 1993). Milk was then collected
at the afternoon milking on the day of treatment and at each
afternoon milking after that, with duplicate composite sample
analyzed for each cow's milk, until residues were below the
detection limit of 0.025 mg/L of the LC method of analysis.
Residues ranged from 8.5 to 17.0 mg/L in the day 1 samples
to 0.23-0.49 mg/L at day 7 and were < 0.05 mg/L in milk
from 4 of 6 animals at day 18. Residues of 0.03 mg/L persisted
in the milk of one animal to day 31, and in another to day
28. Milk samples were also tested using the B. stearothermophilus
test, which gave positive tests up to 21 days following treatment.
Due to the persistence of residues, tilmicosin has not been
recommended for the treatment of lactating dairy cattle.
METHODS OF ANALYSIS FOR RESIDUES IN TISSUES AND MILK
Screening Tests for Tissue and Urine
No results obtained using commercially available
test kits to screen for tilmicosin residues in tissues were
reported in the file provided by the sponsor. The Delvotest
P was applied on milk samples collected in one study, described
above (Patel et al, 1992). Experience in national monitoring
programs suggests that some of the commonly used screening
tests, such as the Swab Test on Premises (STOP), which is
based on the inhibition of the growth of Bacillus subtilis,
will detect residues of tilmicosin in organ tissues at levels
of regulatory interest. Some commercially available tests
designed for the detection of other macrolide antibiotics
may also prove suitable for the detection of tilmicosin residues.
However, no published reports are currently available to demonstrate
this possibility.
Microbiological Assays
A microbiological plate assay for the determination
of tilmicosin in bovine blood serum using Micrococcus luteus,
ATCC 9341, as the indicator organism has recently
been reported (Coleman et al, 1995). The method,
which has an LOD of 0.05 mg/L and an LOQ of 0.08 mg/L, has
not been reported as applied to tissue samples.
Chemical Methods
Several methods using liquid chromatography were
submitted by the sponsor. These include methods for the analysis
of serum, liver, kidney, lung, muscle, fat and injection site
tissues from sheep (Patel et al, 1993) and sheep's
milk (Patel et al, 1992). Analytical methods using
HPLC for the assay of cattle tissues, including liver, kidney,
muscle (Donoho et al, 1988) and fat (Peloso and Thomson,
1988) have also been described. Similar methods have been
applied to swine tissues, including liver, kidney, muscle
and skin/fat (Donoho et al, 1991; Readnour and Darby,
1993). Typically, residues are extracted from tissue with
methanol, partitioned with chloroform and carbon tetrachloride
and analyzed by reverse phase liquid chromatography with UV-detection
at 280 nm and LOD in the 0.005 to 0.01 mg/kg range. Sample
stability was also investigated as part of these studies.
Fortified tissue samples were stored for 2-3 months at -20ˇăC
in the studies on methods for cattle and swine tissues, then
analyzed as part of the method validation. Results were generally
within 10-15 % of recovery values for freshly fortified tissues,
indicating that analyte loss during storage prior to analysis
did not appear to be a major concern. No data were provided
on the stability of incurred residues.
An HPLC method for the simultaneous determination
of the macrolide antibiotics tylosin and tilmicosin has also
been reported (Chan et al, 1994). Following extraction
with acetonitrile and buffer, samples are passed through a
C-18 solid phase extraction cartridge. Tilmicosin is eluted
from the cartridge with 0.1 M ammonium acetate in methanol
and analyzed by reversed phase HPLC with UV-detection at 287
nm. The limit of detection in bovine and porcine muscle and
kidney is reported as 0.01 mg/kg.
APPRAISAL
Tilmicosin is available as an injectable formulation,
administered subcutaneously in cattle and sheep, and as a
medicating ingredient for swine feeds. Reports of studies
provided by the sponsor were well-detailed and most met GLP
standards. Absorption of the injectable formulation is good
in cattle and sheep, with maximum concentrations in blood
observed in 6-12 hours after treatment at the recommended
dose of 10 mg/kg BW. Elimination in rats, cattle, sheep and
swine follows a similar pathway, with the majority of the
residues eliminated in the faeces, but significant residues
are also eliminated in the urine. Radiolabel studies indicate
that approximately 90% of a dose is eliminated within 14-21
days following treatment. Residues are distributed primarily
in the liver and kidneys, with much lower residues found in
normal muscle tissue and fat. Significant residues may remain
at injection sites for some time following treatment, with
2.94 mg/kg found in cattle after 28 days withdrawal in one
study (see Table 7) and 1.53 mg/kg found at 14 days in sheep
(Table 8). Studies in all species reported (rats, cattle,
sheep, swine) identify parent compound as the major residue
found and also indicate that residues are most persistent
in liver, followed by kidney. Based on these results, parent
tilmicosin is recommended as the marker residue, liver is
recommended as the target tissue for monitoring programs,
but kidney is an acceptable alternative. As the major tissue
in trade, however, it is recognized that muscle tissue may
be more readily available for international monitoring. Based
on the depletion data reviewed, it would appear that the most
likely source of detectable residues in a muscle sample might
be from an injection site. Due to the persistence of residues
in milk, tilmicosin is not recommended for treatment of lactating
dairy cattle.
While the methods submitted by the sponsor provided
acceptable sensitivity, they would be regarded as unsuitable
by many regulatory laboratories because of their requirement
for the use of carbon tetrachloride and/or chloroform. In
addition to the safety concerns for laboratory personnel who
may be occupationally exposed to these solvents, disposal
costs are high and availability may in future be limited due
to environmental concerns. A method that does not require
these solvents has been published and appears suitable for
use in a regulatory monitoring program, but results are only
available for kidney and muscle tissue. The reported LOD for
the method is 0.01 mg/kg for parent tilmicosin.
Maximum Residue Limits
In reaching its decision on the MRLs for tilmicosin,
the Committee took into account the following:
-
an ADI of 0-40 m
g/kg of body weight was established, equivalent to a maximum
daily intake of 2400 m g for a 60 kg person;
- the total residues, other than parent compound, were not fully characterized
in the depletion studies and therefore must be considered;
- liver is the appropriate target tissue;
- the primary tissue in international trade is muscle tissue;
- the absence of a radiolabel residue study in lactating sheep;
- the appropriate marker residue in all tissues is the parent compound;
- suitable analytical methods are available for the marker residue;
- available data indicate that the following percentages should be applied
to relate marker residue to total residue in the following
tissues:
- cattle and sheep liver, 5 %;
- cattle kidney, 25%;
- sheep's kidney, 10%;
- swine liver and kidney, 50%;
- muscle and fat (cattle, sheep, swine), 50%.
- milk (sheep), 50%, based on distribution in fat and muscle.
Based on these considerations, the Committee recommended
the following permanent MRL's, expressed as the parent drug:
|
Cattle and sheep: |
liver |
1000
m
g/kg |
|
kidney |
300
m
g/kg |
|
muscle |
100
m
g/kg |
|
fat |
100
m
g/kg |
|
Swine: |
liver |
1500
m
g/kg |
|
kidney |
1000
m
g/kg |
|
muscle |
100
m
g/kg |
|
fat |
100
m
g/kg |
A temporary MRL of 50 m
g/L was recommended for milk from sheep.
Based on the above MRL's which combined with the
conversion factors for sheep to give the highest total residue
and the standard food basket, the following theoretical maximum
daily intake is calculated:
|
- for liver |
1000
m
g/kg x 0.10 kg/0.05 = |
2000 m
g |
|
- for kidney |
300
m
g/kg x 0.05 kg/0.10 = |
150 m
g |
|
- for muscle |
100
m
g/kg x 0.30 kg/0.50 = |
60 m
g |
|
- for fat |
100
m
g/kg x 0.05 kg/0.50 = |
10 m
g |
|
- for sheep milk |
50
m
g/L x 1.5 L/0.50 = |
150 m
g |
|
Total |
2370 m
g |
The Committee wishes to draw attention to the possibility
that a potential exists for residues in excess of MRLs for
muscle tissue to exist in injection sites at withdrawal times
necessary to be in compliance with the above MRLs.
REFERENCES
Chan, W., Gerhardt, G.C., and Salisbury, C.D.C.,
(1994). Determination of tylosin and tilmicosin residues in
animal tissues by reversed-phase liquid chromatography. J.
AOAC Int. 77: 331-333.
Cochrane, R.L., and Thomson, T.D.,
(1990). Toxicology and pharmacology of tilmicosin following
administration of subcutaneous and intravenous injections
to sheep. Research Report T5C768908, Sponsor Submitted.
Coleman, M.R., Peloso, J.S., and Moran, J.W.,
(1995). Microbiological plate assay for determination of tilmicosin
in bovine serum. J. AOAC Int. 78: 659-662.
Donoho, A.L., (1988). Comparative metabolism of 14C-tilmicosin
in cattle
and
rats. Research Report ABC-0395, Sponsor Submitted.
Donoho, A.L., and Thomson, T.D.,
(1988). 14C-Tilmicosin
balance-excretion study in swine. Research Report ABC-0409,
Sponsor Submitted.
Donoho, A.L., Peloso, J.S., and Thomson, T.D.,
(1988). 14C-Tilmicosin
tissue residue study in cattle. Research Report ABC-0383,
Sponsor Submitted.
Donoho, A.L., Cochrane, R.L., and Helton, S.L.,
(1991). 14C-Tilmicosin
tissue residue decline study in swine dosed orally at a level
of 600 ppm in feed. Research Report T5C759101, Sponsor Submitted.
Donoho, A.L., Helton, S.L., Darby, J.M., Sweeney, D.J., Occolowitz,
J.L., and Dorman, D.E., (1992).
Tilmicosin metabolism study in tissues and excreta of pigs
fed 400 ppm 14C-tilmicosin.
Research Report T5C759201, Sponsor Submitted.
Donoho, A.L., and Kennington, A.S.,
(1993). Tilmicosin metabolite study with rat excreta. Research
Report T5C759302, Sponsor Submitted.
Giera, D.D., Herberg, M.S, and Thomson, T.D.,
(1986a). 14C
EL-870 balance excretion and tissue residue in a steer. Research
Report ABC-0299, Sponsor Submitted.
Giera, D.D., Herberg, M.S, Klink, P.R., and Thomson, T.D.,
(1986b). 14C
EL-870 tissue residue decline study and balance excretion
study in cattle. Research Report ABC-0340, Sponsor Submitted.
Giera, D.D., and Peloso, J.S.,
(1986). Characterization of radioactive residues in cattle
tissues following therapeutic dose of 14C
EL-870. Research Report ABC-0353, Sponsor Submitted.
Giera, D.D., and Thomson, T.D.,
(1986). Preliminary 14C
EL-870 balance excretion and tissue residue in swine. Research
Report ABC-0305, Sponsor Submitted.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A., and Cameron,
D.M., (1993). The metabolism and residues of 14C-tilmicosin
following subcutaneous administration to sheep. Research Report
HRC/LLY 36/930447, Sponsor Submitted.
Helton-Groce, S.L., Thomson, T.D., and Readnour, R.S.,
(1993). A study of tilmicosin residues in milk following subcutaneous
administration to lactating dairy cows. Can. Vet. J.
34: 619-621.
Patel, R.K.P., Parker, R., Simmons, H.A., Hassanali, H.T.,
Brown, A.J., and Bucknall, A.J., (1991). Tilmicosin: pharmacokinetics in sheep. Research
Report CVLS5/91/A, Sponsor Submitted.
Patel, R.K.P., Parker, R.M., Walker, A.M., McLaren, I.M., Bucknall,
A.J., and Hopkins, I.G., (1992).
Tilmicosin: milk depletion study in sheep. Research Report
CVLS3/92, Sponsor Submitted.
Patel, R.K.P., Parker, R., Walker, A.M., Bucknall, A.J., and
Hopkins, I.G., (1993). Tilmicosin: metabolism and residues of 14C-tilmicosin
following subcutaneous administration in sheep: HPLC analysis
of plasma and tissues for the parent compound. Research Report
CVLS4/92, Sponsor Submitted.
Patel, R.K.P., Parker, R.M., Phillips, J.B., Walker, A.M.,
Coombes, R., and Lockyer, J.F., (1995). Tilmicosin: residues in sheep after its subcutaneous
administration. Research Report CVLS/23/95, Sponsor Submitted.
Peloso, J.S., and Thomson, T.D.,
(1988). Tilmicosin tissue residue decline study in cattle.
Research Report AAC8701, Sponsor Submitted.
Readnour, R.S., and Darby, J.M., (1993). Tilmicosin
tissue residue decline study in swine. Research Report T5C619301,
Sponsor Submitted.
Thomson, T.D., (1989a). Serum tilmicosin profiles
following a single 10 mg/kg administration of the proposed
tilmicosin bovine parenteral formulation in neonatal calves
in several anatomical sites. Research Report T5C768804, Sponsor
Submitted.
Thomson, T.D., (1989b). Serum tilmicosin profiles
following a single 10 mg/kg administration of the proposed
tilmicosin bovine parenteral formulation in feedlot-type cattle
in several anatomical sites. Research Report T5C768805, Sponsor
Submitted.
Thomson, T.D., Darby, J.M., Moran, J.W., Tonkinson,
L.V., (1993). Serum and lung tilmicosin
concentration in swine following dosing with tilmicosin fortified
feed. Research Report T5CAX9302, Sponsor
Submitted. |
